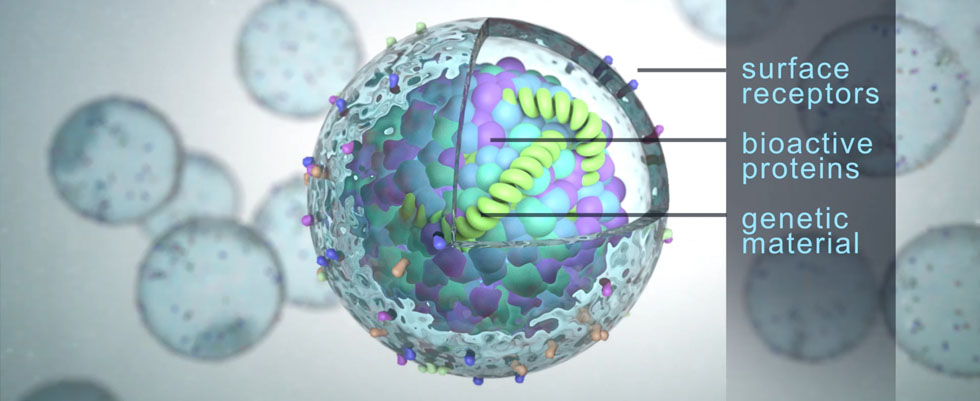
CustomExTM: Developing customisable Exosomes as a targeted delivery platform for complex drug modalities
What are the advantages of CustomExTM
- A delivery mechanism for a variety of payloads including nucleic acids, proteins, and gene editing technologies.
- ReNeuron uses its conditionally immortalized induced pluripotent stem cell (iPSC) platform to make allogeneic tissue cells of choice. These then have the potential to produce Exosomes with tissue specific targeting ability.
- Technologies supported by an extensive Intellectual property portfolio
Legacy Assets available for Partnering
Conditionally immortalized Induced Pluripotent Stem cell (iPSC) platform
- For the rapid development of new allogeneic cell lines as cell-based therapeutics or for the production of exosomes with specific tissue targeting.
hRPC Program in Retinitis Pigmentosa
- Our Human Retinal Progenitor Cell line (hRPC) has been dosed in patients in a Phase 1/2a clinical program in the US. The Company intends to out-license its RP program (outside of China).
CTX Stroke Program
- ReNeuron’s immortalized neural progenitor cell line (CTX) completed a Phase 2 b study in the US as a therapy for the treatment of patients left disabled by a stroke.
- This program continues through its partnership with Fosun Pharma in China and is available for out-license outside of China.
ReNeuron is incorporated in the UK with offices in the UK and the US. Our shares are traded on the London AIM market under the symbol RENE.L